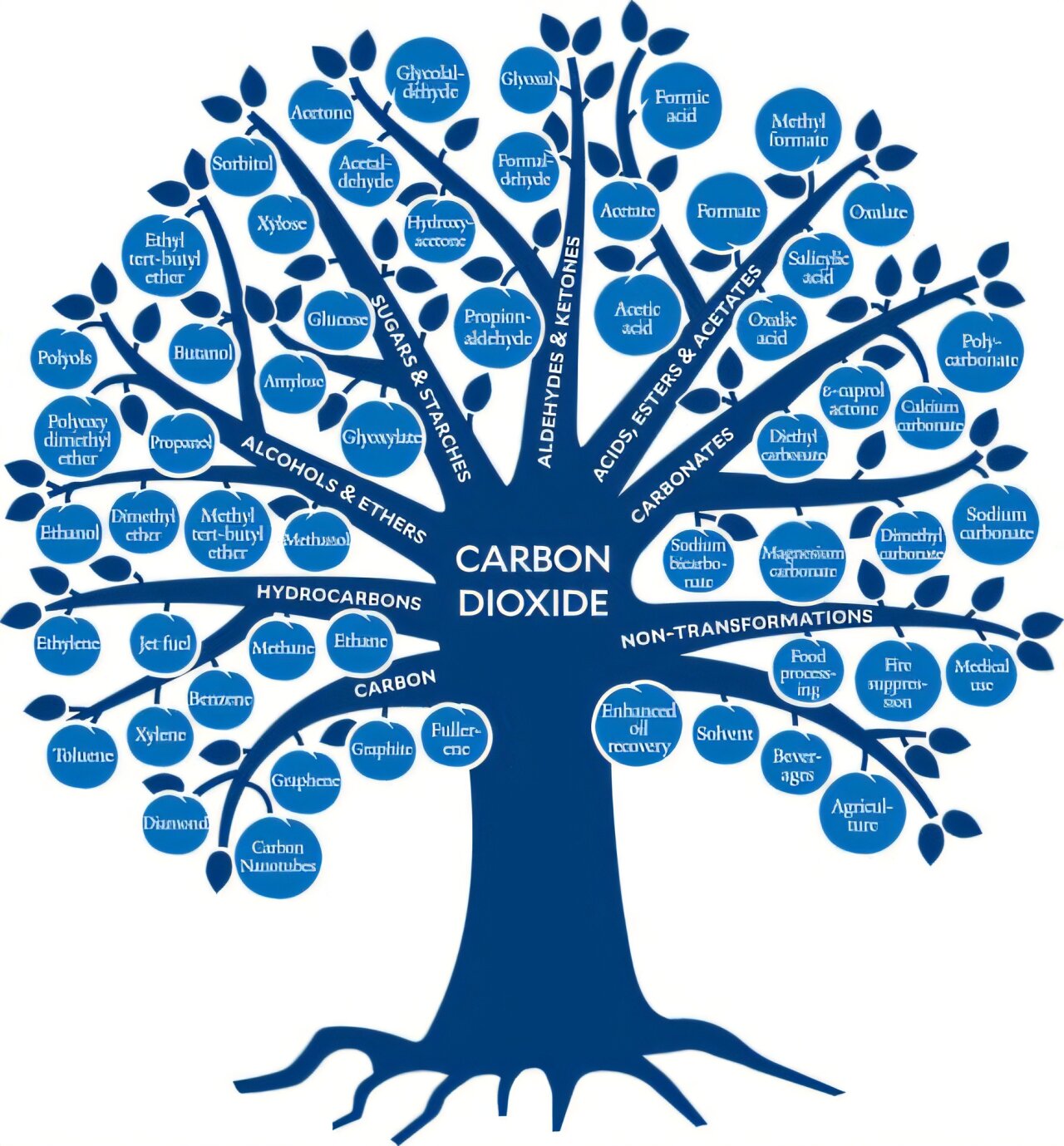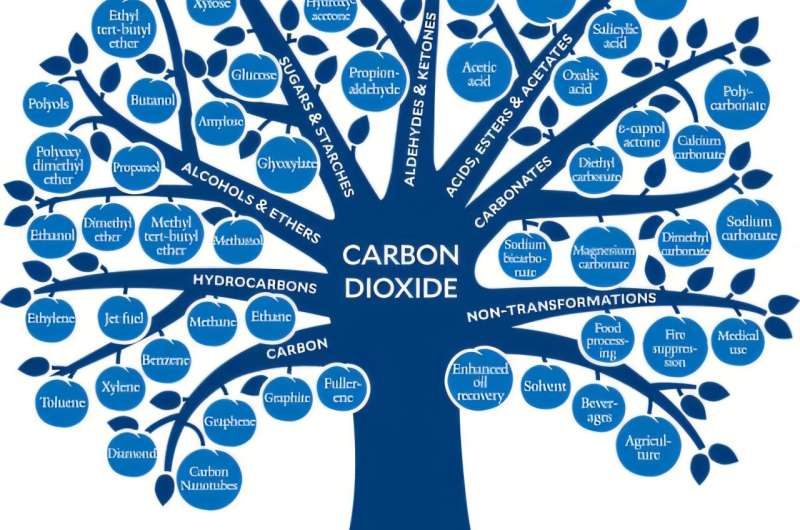
The CO2 Baum emphasizes the classes (branches) of connections (fruits), which were made from carbon dioxide from the laboratory scale to commercial scale. Credit: ACS Sustainable Chemistry & Engineering (2024). DOI: 10.1021/Acsuschemeng.4C07582
Carbon dioxide emissions (CO₂) missions contribute to a significant contribution to the climate crisis, and while the world works to reduce emissions, chemists and engineers also work on how the carbon dioxide already emitted records and uses.
As soon as the researchers have been released as impractical, they develop methods to convert CO₂ into a wide range of valuable products such as consumer goods, industrial materials or chemicals. This progress is illustrated by “The Co₂ Tree”, which shows the wide range of products that can be achieved by using CO₂ using a tree metaphor.
Hanno Erythropel, a research scientist at the Yale Center for Green Chemistry and Green Engineering at the Yale School of the Environment, is part of an international team that has published the article “The CO₂ Tree: The potential for carbon dioxide outlets” in “In” In “In” In “in” ACS Sustainable Chemistry & Engineering. He recently spoke to YSE News about how chemical CO₂ conversion can contribute to alleviating the climate crisis and why it is not a silver ball.

Hanno Erythropel in the laboratory. Credit: Mara Lavitt
How does the chemical conversion of CO₂ work into products?
From a chemical point of view, CO₂ is a molecule like any other, and chemists convert molecules to create the materials required for our modern society. What makes CO₂ special is that it is usually the end product of combustion and related processes and is considered “low energy”, since you can convert them back into useful materials, you need energy and usually hydrogen to remove oxygen atoms from it.
This is exactly what chemist striving for: We try to develop effective ways to convert a molecule into another. If we look at the CO₂ tree that we have created for the manuscript, we organize the fruits – the materials already available by CO₂ – from the most reduced (all oxygen) as in fuels or graphite on the left, up to the AM The strongest oxidized (most oxygen removed) as in baking powder, sodium bicarbonate, right.
Can the conversion of CO₂ to help us to reach our climate goals?
We know that we have to reduce our CO₂ production, which results from fossil resources, but the CO₂ in the atmosphere can also serve as a resource, especially because we have to find and use alternative carbon sources for the currently used fossil fascile fossils. If chemists want to do their part to combat climate change, this is a good way to say: “Well, how can we convert CO₂ into something useful?”
The materials and chemicals for our society of CO₂ and not made of fossil resources is an inherently circular approach: after all, chemicals and materials become CO₂ again, which is again a resource. If we use this in the manuscript, we essentially harvest such a renewable energy into the material world.
We sincerely hope that the list presented will soon be outdated, since research and development in the area, which is guided by teams by creative chemists and engineers, have made many more chemicals and materials made of CO₂ accessible.
Are there any concerns that people see them and believe that we do not have to reduce CO₂ emissions because we can make products out of it?
This is a very valid point that was also increased during the review process. Let us be clear: CO₂ usage will only be a factor, apart from the reduction of fossil emissions and the carbon cover to tackle the climate crisis.
We really want to be clear that what we have described here here, the energy balance and other life cycle factors. Ignoring these important factors can cause the right things to be done incorrectly. In other words, the conversion of waste into valuable products, but at the same time unintentional consequences.
The goal of this manuscript is therefore not to assess what should be done or not, but rather to raise awareness of the fantastic work of converting CO₂ into something useful that is already available. When implementing such reactions, we have to make sure that we do the right things right. For example, if more CO₂ is generated and released than was installed during the process or a toxic catalyst was used, the problem could possibly have been worsened. So there is a need to be thoughtful.
What do you hope will be the greatest snack from the study?
The great hope is that chemists, scientists, supervisory authorities and everyone interested to see this and say: “Oh, wait a minute. It is possible to convert CO₂ into useful materials!” In the manuscript, we have given examples of reactions for different materials and provided resources for those who are interested in examining this in more detail.
While we outline in the manuscript and our own professor Paul Anastas: “We can only hope that this list of the possible products we have provided have made a new tree picture to record the progress in the field.”
Further information:
Heather O. Leclerc et al., Co2 Baum: The potential for carbon dioxide usage paths, ACS Sustainable Chemistry & Engineering (2024). DOI: 10.1021/Acsuschemeng.4C07582
Provided by Yale University
Quote: Questions and answers: CO₂ for consumer goods and industrial materials (2025, February 6) accessed on February 6, 2025 by https://phys.org/news/2025-02-qa-harning-consumer-goods- industrial.html
This document is subject to copyright. Apart from a fair handling of the purpose of the private study or research, no part may be reproduced without a written approval. The content is only provided for information purposes.