Researchers have unveiled a fast, environmentally friendly technique that could revolutionize the production of lithium batteries.
The method, developed by a group at Pohang University of Science and Technology (POSTECH) in South Korea, enables complete solvent recovery to produce sustainable materials while improving electron and ion transit and lithium storage capacity.
Experts say the discovery solves a long-standing problem in battery design by achieving nanoscale homogeneity between metal oxides, which effectively store lithium, and carbon-based or MXene materials, which enable rapid electron flow.
Recently, Korean researchers developed an ultra-thin silver ion coating that prevents dendrite formation in lithium metal batteries, improving the safety, durability and commercialization potential for next-generation energy storage.
Next generation anode design
Conventional manufacturing methods often fail to maintain nanoscale uniformity in the production of lithium electrodes. The slow evaporation of the solvent leads to particle clumping and pore collapse, weakening the precise structures required for efficient energy storage.
Evaporation-induced self-assembly (EISA), in which metal precursors and polymers form mesoporous frameworks as the solvent dries, is a self-assembly technique that researchers have studied to address this problem. EISA is sluggish, difficult to regulate and prone to uneven mixing. According to Nanowerk, the addition of conductive materials such as MXenes or carbon nanotubes can worsen the problem because they come off when metal oxides dry and disrupt electrical wiring.
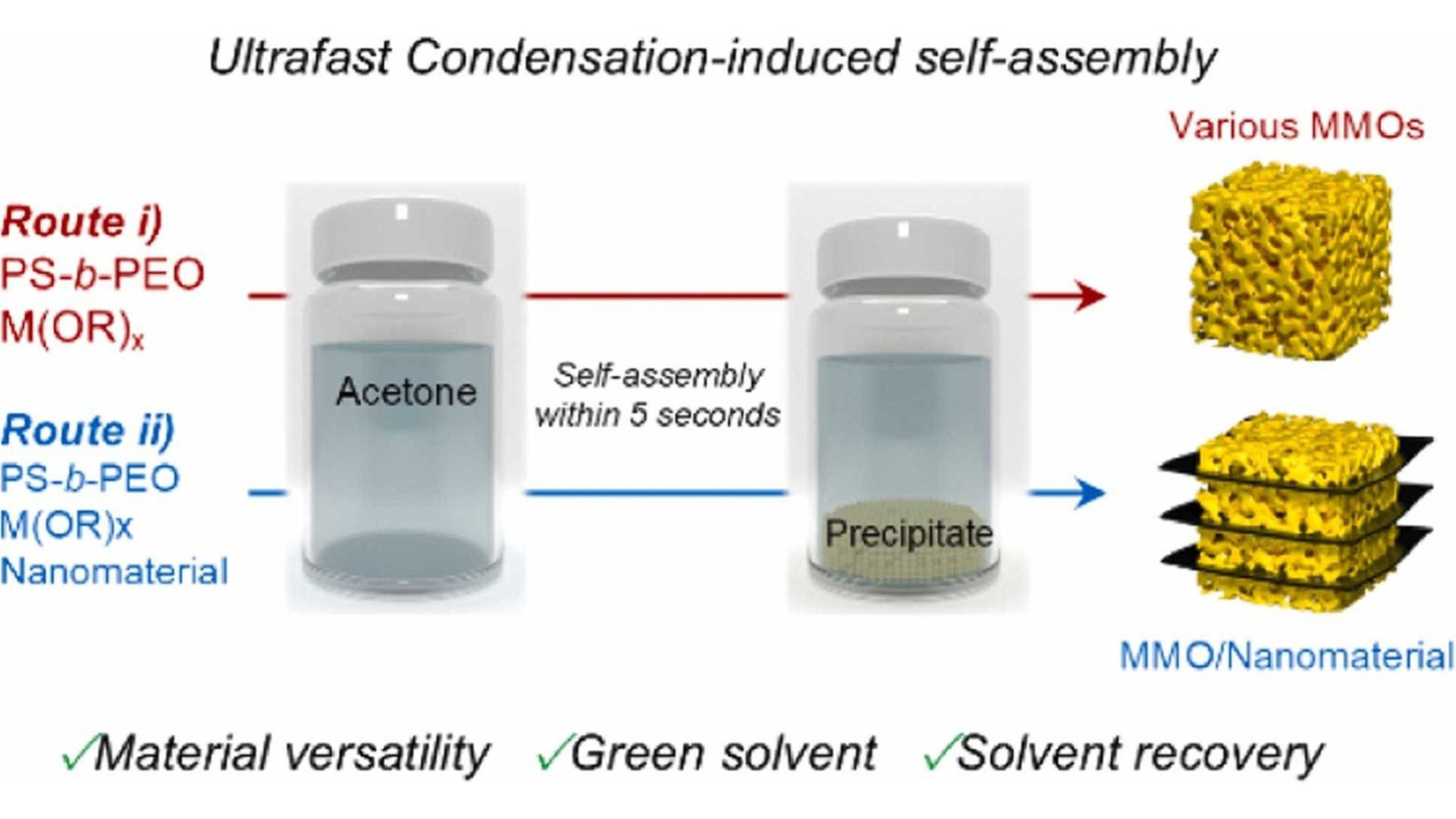
Scalability is also compromised by the use of hazardous, non-recyclable solvents in many processes. Achieving rapid, consistent self-assembly in solution without evaporation remains a significant difficulty despite advances in block copolymer templates, metal alkoxide chemistry, and nanomaterials.
POSTECH research offers a solution and reports a rapid self-assembly method called condensation-induced self-assembly (CISA). Instead of slow solvent drying, it uses metal alkoxide reactions to organize materials within seconds, creating uniform, porous metal oxides with well-mixed 1D and 2D conducting nanomaterials for better lithium storage.
“Our approach uses the condensation reaction of metal alkoxides as a driving force for self-assembly, enabling the formation of uniform mesoporous metal oxides within a few seconds,” Jin Kon Kim, professor at POSTECH and research leader, told Nanowerk.
Accelerated material production
In the CISA process, a block copolymer (PS-b-PEO) dissolved in strongly acidic acetone triggers rapid hydrolysis and condensation of metal alkoxides such as niobium ethoxide. This chemical reaction causes the solution to form micelles, which quickly assemble into a soft gel and later solidify into a porous oxide structure. The reaction completes in about five seconds – much faster than evaporation-controlled methods – and binds all components before separation occurs.
According to the team, the resulting materials, which include oxides of tungsten, titanium and niobium, have large surface areas, stable crystalline grains and regular pores – all essential for rapid ion transport. Because acetone is recyclable and non-contaminated, the process allows for complete solvent recovery, promoting low-waste, environmentally friendly production.
CISA incorporates conductive nanomaterials such as MXenes and carbon nanotubes during assembly to ensure uniform distribution across the oxide matrix without pore collapse. The resulting niobium oxide-MXene composite outperformed traditional evaporation-based materials in lithium-ion battery performance, producing 163 mAh/g at 1 A/g and maintaining 115 mAh/g after 1,000 cycles. Furthermore, impedance experiments confirmed increased ion diffusion and electron mobility.
Researchers claim that this reaction-controlled method enables scalable, energy-efficient synthesis of nanostructured materials by replacing slow physical drying with fine chemical control. Through rapid, environmentally friendly synthesis, CISA has the potential to produce not only lithium-ion batteries but also high-performance catalysts, sensors and other functional materials.
The details of the team's research were published in the journal Nano energy.
